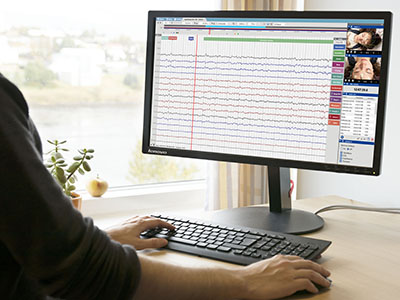
Stratus EEG
Customer
Stratus EEG is an advanced EEG acquisition, review and patient database solution in use worldwide for diverse applications such as clinical, routine, ICU, NICU, ER, home recording, research and veterinary. The brand draws on decades of experience in engineering for the EEG field on behalf of Kvikna's medical division.
The Challenge
EEG monitoring gives a wealth of information about patient condition which can save lives, however, the expertise of trained EEG experts is not always on hand locally.
The Solution
Kvikna introduced a game-changing cloud services solution that allows recording to take place in one location, while simultaneous review can take place anywhere with an internet connection. The result is not only more secure than previous systems which needed to be backed up using traditional methods, but improves data integrity by being consistently up-to-date and synchronized system wide. The solution has been developed in strict accordance with the following regulatory procedures and standards:
- Quality Management System for Medical Devices (ISO 13485:2016)
- Medical Device Directive (93/42/EEC)
- FDAs 21 CFR 820
- Medical Software Life Cycle Processes (IEC 62304:2006)
- Medical Device Risk Management (ISO 14971:2012)
- HIPAA
- GDPR
The Technology
Stratus EEG acquires signals from an amplifying device connected to a patient’s head via electrode sensors. The software displays the signals as EEG traces, alongside synchronous HD video from 1 or 2 cameras, in a user-centered interface developed for a variety of touch screen or mouse-driven devices. Stratus EEG is built on Service Oriented Architecture – the signals are processed server side, which greatly reduces requirements on the client side.
For more information about Stratus EEG, visit their
website.